The OpenStudyBuilder: Accelerating Clinical Trials Through Metadata-Driven Design
Planning clinical trials is complex and time-consuming – from protocol development to final implementation, months can pass. But what if this process could be significantly more efficient and faster? This is where the OpenStudyBuilder (OSB) comes in. As an innovative tool, it enables standardized, collaborative, and automated study protocol creation. In this blog, you'll learn how the OSB works, its benefits, and why it could play a key role in shaping the future of clinical research.

The Cost of Delay: Current Challenges in Clinical Trials
Time is critical in clinical trials. The pharmaceutical industry faces mounting pressure to accelerate time-to-market for new therapies while maintaining high-quality standards in study execution. Despite technological advancements, study startup processes in particular protocol development and study design are plagued by inefficiencies.
Current industry statistics highlight the severity of these challenges:
- Clinical trials face delays in 85% of cases due to prolonged protocol creation and suboptimal study designs
- The study startup phase requires an average of eight months, significantly impacting trial timelines and patient recruitment
- Protocol amendments occur in 57% of trials, with each amendment incurring costs of approximately $535,000
- The financial impact of delays is substantial, with daily costs potentially ranging between $600,000 and $8 million for drug development
These inefficiencies often stem from inconsistent or overly manual business processes, which can lead to challenges in execution and compliance. To address this, business processes must be optimized continuously. Technology plays a crucial role in supporting this transformation by providing integrated, scalable systems that eliminate silos and enable seamless data flow. The key lies in adopting integrated solutions that streamline clinical trial processes, enhance collaboration, and drive efficiency without compromising on sustainability or security.
A key element in achieving this optimization is the use of metadata. In clinical trials, metadata refers to structured data that describes essential elements of the study, such as Study IDs, objectives, eligibility criteria, and endpoints. Rather than relying on static documents, this data is stored in a dynamic, reusable format that can be shared and updated across multiple systems. This metadata-driven approach ensures that all stakeholders have access to the most current and accurate information, enhancing consistency, reducing redundancy, and improving overall efficiency.
The OpenStudyBuilder (OSB) is a prime example of a clinical tool designed with these principles in mind. By utilizing a metadata-driven approach, the OSB accelerates study startup and simplifies the management of clinical trials, addressing many of the inefficiencies faced in traditional processes.
The OpenStudyBuilder: A Metadata-Driven Approach
The OpenStudyBuilder is an open-source platform developed by Novo Nordisk that represents a paradigm shift in clinical trial design and execution. As part of the CDISC Open Source Alliance (COSA), this innovative solution addresses long-standing industry challenges through a comprehensive, metadata-driven approach.
The platform's architecture consists of three primary components that work together seamlessly:
- The OpenStudyBuilder App: A web-based user interface providing intuitive study design and metadata management capabilities.
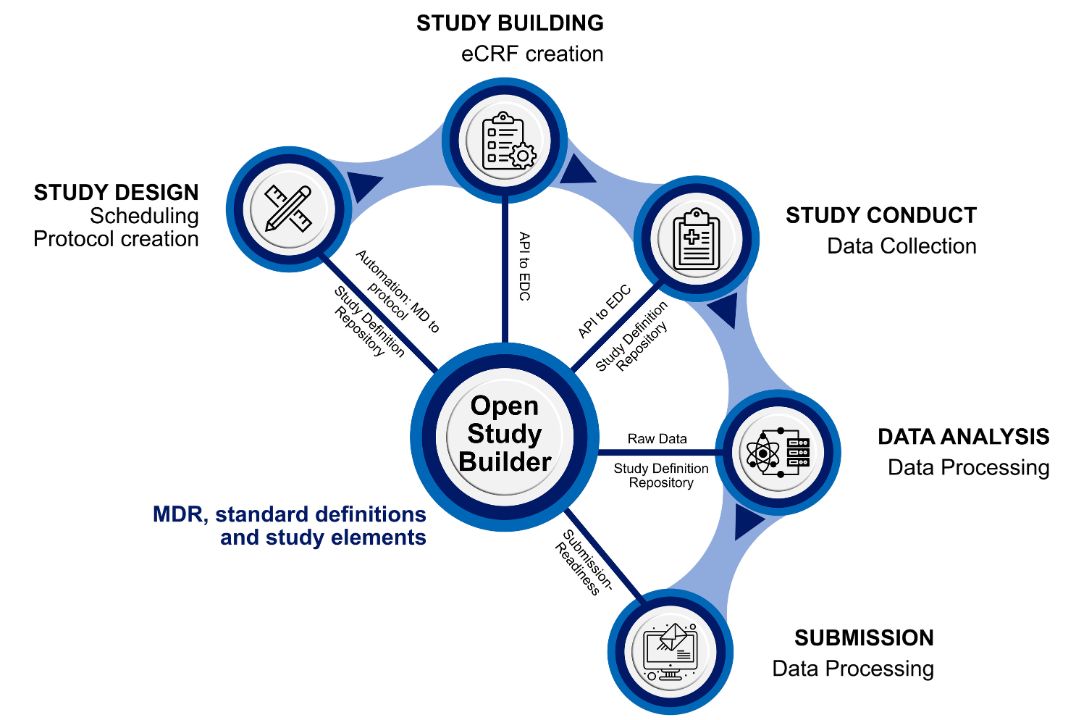
- The Clinical Metadata Repository (MDR): At the heart of OSB is a Clinical Metadata Repository built on a graph database (Neo4j), which organizes study data by linking information in a network-like structure. This makes it easier to find relationships between data points and enables faster, more efficient querying.
- The Integration Layer: A Python-based FAST API (application programming interface) framework that ensures robust system integration including but not limited to Electronic Data Capture (EDC) systems.
At its core, OSB leverages these components to establish a centralized repository that serves as a single source of truth for the study-related metadata. This ensures consistency across the entire clinical trial lifecycle, from protocol development and Case Report Form (CRF) design to submission. The integration layer plays a crucial role here, enabling integration with existing clinical trial systems and supporting data exchange across the entire research ecosystem.
The open-source nature of the OpenStudyBuilder brings significant advantages to the clinical research community. It fosters continuous innovation and collaboration, allowing organizations to customize and extend the platform based on their unique requirements while benefiting from collective contributions and improvements from other industry stakeholders. The transparency of the open-source model promotes higher adoption rates and facilitates regulatory acceptance.
As with any evolving platform, organizations considering OSB should plan their implementation thoughtfully. While some advanced features are still under development, the core functionality is robust and production-ready. The platform's approach focused on standardization makes integration with existing systems straightforward, though it requires initial configuration effort. As an open-source solution, OSB offers organizations full control over their implementation, supported by a growing community of developers and users. Due to its open-source nature, the OSB requires a different support model than traditional vendor-backed solutions. Instead of relying on a single provider, organizations can benefit from community-driven resources.
Successful implementation of OSB requires commitment to maintaining and potentially enhancing the platform - an investment that typically pays off through increased efficiency and data quality. Organizations can either build internal expertise or partner with experienced consultants who understand both the clinical research domain and the platform. The result is a powerful and flexible solution that can significantly streamline clinical trial processes and improve data consistency across the entire study lifecycle, while providing full control over the implementation and future development.
Aligning with Industry Trends: OSB's Strategic Role
The clinical trial industry is witnessing a significant shift towards advanced technologies to enhance efficiency. OSB aligns perfectly with three major trends that are reshaping the future of study startup:
1. Advanced Study Design Tools and Automation
OSB harnesses the power of metadata repositories which can be combined with machine learning (ML) or other tools to facilitate automation in protocol creation, reducing effort and time needed. ML algorithms could be used to analyze historical protocols to recommend optimal study designs, refine inclusion/exclusion criteria, generate language translations, and automate protocol drafting. In addition, meta data can automatically be transferred from the repository to the study protocol or vice versa. These streamlined approaches help organizations minimize costly revisions and accelerate study startup processes.
2. Integrated IT Ecosystems
Modern clinical IT landscapes have evolved beyond standalone systems into deeply interconnected platforms and will continue to become increasingly integrated landscapes. OSB exemplifies this evolution by enabling end-to-end connectivity across study design, execution, and regulatory submission. Through direct metadata links to downstream systems—including CRF libraries and EDC platforms like Oracle Clinical One and, in the future, Veeva Vault—OSB enables seamless data flow while minimizing duplication and errors.
3. Standardization as the Foundation for Integration
Standardization is a key enabler of efficient and interoperable clinical trial data flow. A consistent data structure is essential to facilitate seamless data exchange between systems, reducing redundancy and ensuring a smooth study setup. OSB actively supports industry-wide initiatives by adhering to standards such as CDISC and TransCelerate and supporting the M11 Template, which provides a structured, standardized protocol framework.
Beyond standard templates, OSB leverages metadata-driven workflows to replace static documents with reusable, structured data components. This approach enhances adaptability and scalability, allowing study definitions to be efficiently managed across multiple platforms.
While OSB provides clear technical advantages, its real value lies in how it benefits the various stakeholders involved in clinical trials. Sponsors can accelerate study planning by leveraging structured metadata, which not only increases efficiency but also allows key study elements to be reused across multiple trials, minimizing redundant work. Contract Research Organizations (CROs) benefit from improved collaboration with sponsors, as OSB ensures consistent data formats and facilitates the reuse of study designs, leading to smoother operational workflows. Regulatory bodies also stand to gain, as OSB promotes standardization and adherence to frameworks like CDISC, making regulatory submissions more efficient and reducing compliance risks.
By aligning the needs of these stakeholders, OSB helps create a more integrated and streamlined approach to clinical trial design, ultimately improving efficiency across the entire research landscape.
Current Capabilities and the Road Ahead
The OpenStudyBuilder has established itself as a robust study design tool with an integrated metadata repository. A significant milestone in its development was the successful pilot integration with Oracle's Clinical One EDC system, showcasing its potential for seamless data exchange. Building on this, further integrations with other EDC systems are planned, in particular Veeva EDC. Currently, OSB offers comprehensive support for standardized protocol development, sophisticated metadata management and reuse, and strict adherence to industry standards such as CDISC and SNOMED.
However, OSB continues to be developed. A crucial upcoming enhancement is the introduction of advanced automation features that will streamline metadata transfer for protocol creation. While this functionality is expected to roll out soon, additional automation possibilities—such as integrations with structured content management systems and AI-driven solutions—remain in development.
How OSB's Metadata Approach Reshapes Clinical Research
A metadata-driven approach goes beyond mere process optimization. Instead, it fundamentally changes how clinical trials are designed, conducted and managed. By centrally managing and reusing structured trial data, the industry can become more efficient, transparent for all participating stakeholders and regulatory robust in the long term. In an increasingly data-driven environment, OSB not only enables better integration with existing systems, but also lays the foundation for future innovation. In the long term, for example, AI-supported analyses based on metadata could help to plan studies more efficiently or automatically adapt protocols to regulatory requirements.
Getting Started with OSB
Integrating OSB into existing IT architecture is a complex process that requires strategic planning and expertise. In particular, the implementation of a metadata-driven system into an existing clinical infrastructure poses significant but rewarding challenges.
At Inconsult, we guide you through this transformation process with comprehensive support. Our approach is based on your business goals as well as the specific requirements of clinical trials.
We help you to optimize your processes and accompany the cultural change towards integrated, metadata-driven workflows. We ensure a seamless connection to your existing clinical systems and minimize disruption to ongoing studies. By integrating with leading EDC solutions such as Veeva EDC or Oracle’s Clinical One, we enable smooth data exchange. We further assist with the implementation of automatization features, streamline your processes and reduce manual steps over time. Our experienced project management team supervises the entire implementation using both agile and traditional methodologies, ensuring a successful transition that meets your timeline and quality requirements.
Our implementation approach helps organizations realize the benefits of OSB while managing risks and maintaining operational stability. Whether you're beginning your metadata-driven transformation or expanding an existing implementation, INCONSULT offers the practical expertise to support your success.