A Success Story from a German Biopharmaceutical Giant
Revolutionizing Regulatory Documentation with AI
March 28, 2024
- The Challenge
In the dynamic world of biopharmaceuticals, managing regulatory documentation is a Herculean task, often bogged down by legacy systems and inefficient processes. However, a major German biopharmaceutical company has recently made a groundbreaking leap, employing Artificial Intelligence (AI) to streamline their document management system. This blog delves into their transformative journey.
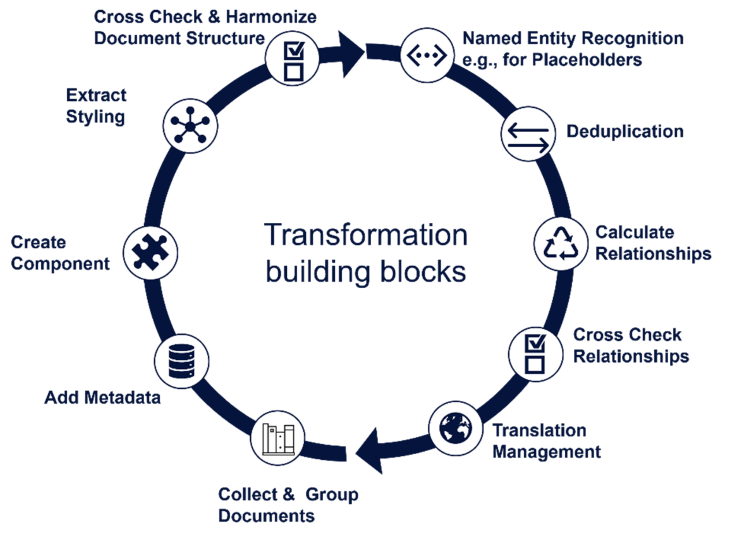
The key challenge was the migration of legacy data from simple formats like PDF and Word into sophisticated Structured Content Management (SCM) systems. This transition necessitated the dissection of legacy documents into logical components, aligning them with ontologies to create compliant, new submissions.
The project was ambitiously named "Intelligent Content Association in Regulated Documents." The goal was to use AI - particularly heuristic and machine learning algorithms - to fragment and intelligently link parts of regulatory documents such as CCDS, LPIs, and eCTD M3 documents.
- Solution Description
The solution hinged on Large Language Models akin to ChatGPT, employing advanced NLP techniques to:
-
- Cross-reference and contextualize information within documents.
- Identify semantically similar content even with varied wording.
- Summarize key information, stripping redundancy.
-
- Process Steps
- Document Analysis: Microsoft Word files and associated metadata were analyzed for content insights.
- Component Extraction: Key components were isolated for in-depth analysis and reuse.
- Semantic and Structural Understanding: Detailed understanding of content elements and their usage was established.
- SCM System Integration: Content and metadata were incorporated as individual components into the SCM system.
- Reuse Analysis: Large Language Models were used to compute and optimize component reuse, enhancing efficiency and reducing redundancy
- Results & Achievements
- The AI algorithm displayed impressive accuracy, especially in simpler products, with a 95% real positive rate.
- In more complex cases, the accuracy remained high at 82% for real positives.
- This approach significantly reduced manual effort by 50%-70%.
- Enhanced change management and improved global documentation oversight were notable outcomes.
- Conclusion
This success story is a testament to the power of AI in revolutionizing documentation processes in the life science industry. It not only signifies a major step in efficiency and compliance but also opens doors for further technological advancements in the field.
Are you intrigued by the potential of AI in regulatory documentation?
Reach out to Stefan Hoferer for more information and discover how these innovative solutions can transform your organization's documentation processes.